Osmosis: Permeable to Solute, Impermeable to Solute
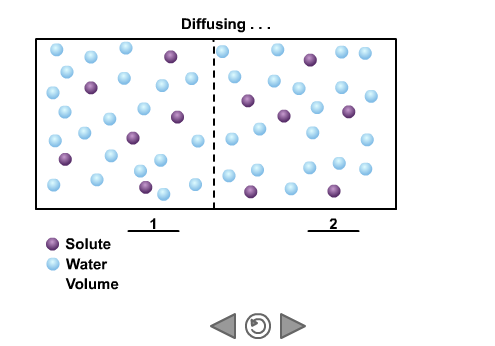 Why does the concentration of a solute cause the movement of water through osmosis? Water is unusual in that it can cross a non-polar membrane but also react and form solutions with ions and polar molecules that cannot cross the same membrane. When water molecules react with a solute they are no longer free to cross the membrane. The more solute, the fewer molecules left that are free to diffuse across the membrane. Therefore a highly concentrated solution (one with lots of solute) has a low effective concentration of water. Therefore water will tend to diffuse across a membrane from areas of low solute concentration to areas of high solute concentration – until the concentration of the solution is equal on both sides. This is only true if the solute cannot cross the membrane.
Why does the concentration of a solute cause the movement of water through osmosis? Water is unusual in that it can cross a non-polar membrane but also react and form solutions with ions and polar molecules that cannot cross the same membrane. When water molecules react with a solute they are no longer free to cross the membrane. The more solute, the fewer molecules left that are free to diffuse across the membrane. Therefore a highly concentrated solution (one with lots of solute) has a low effective concentration of water. Therefore water will tend to diffuse across a membrane from areas of low solute concentration to areas of high solute concentration – until the concentration of the solution is equal on both sides. This is only true if the solute cannot cross the membrane.
Categories and tags of the game : Educational, Science, Single Player
💡 Dato Tecnológico
La comparativa de tarifas de fibra óptica es esencial para gamers. Buscar proveedores de internet que ofrezcan conexión simétrica garantiza la mejor estabilidad en partidas online.